Ensuring FMD compliance with a quality coding and marking provider
22nd November, 2019
The Falsified Medicines Directive (FMD) is a range of staggered regulations first brought into force by the EU in 2013.
It aims to protect people from counterfeit and harmful medicines, which can include wrong or low quality ingredients, inaccurate doses, or those that have been fraudulently mislabelled or packaged.
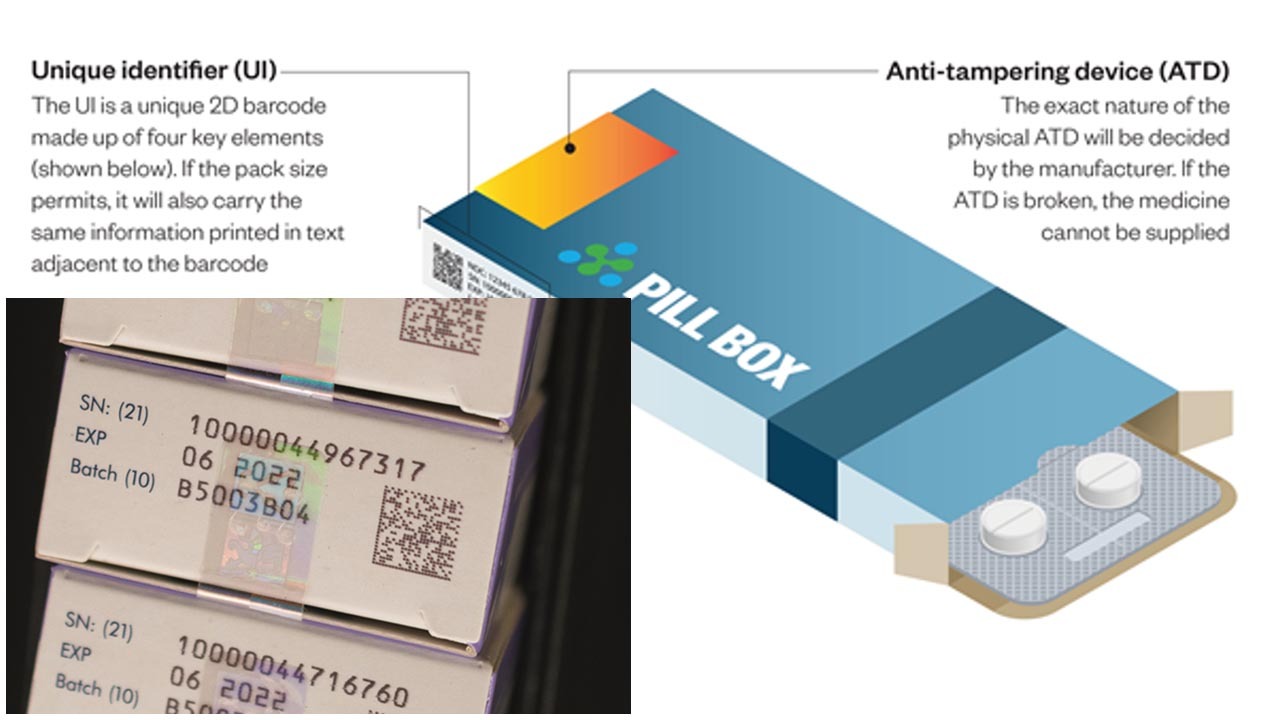
Since 9th February 2019 all medicine sold in the EU must include two safety identifiers. These are a Unique Identifier (UI), a barcode which can be scanned, identifying and tracking the product down the supply chain to the point of purchase, and an Anti-Tampering Device (ATD), which shows if the medicine has been opened at any point.
If at any point the UI is incorrect or ATD is broken the medicine must be decommissioned and cannot be given to the public.
Credit: NHS – https://digital.nhs.uk/service...
It aims to secure the manufacturing and distribution of medicines across the EU by ensuring all medicines are correctly identified and have not been tampered with, guaranteeing the legitimacy of products for the end users.
This has consequences for pharmacies as it dictates that medicines need to be scanned at the point of purchase. This means all pharmacies will legally need to purchase a hand held scanner and incur the cost of this to ensure FMD compliance.
However the costs so far have been prohibitive with under half of pharmacies complying with the rules since they were brought in at the beginning of the year. Many of these are waiting to see how Brexit plays out before they commit to spending, as there is no certainty that the EU initiative will be enshrined in UK law.
The FMD legislation, if it is brought into UK law post-Brexit, makes coding and marking of paramount importance for pharmaceutical manufacturers as any mistakes with the UI barcode, including poor print quality, can lead to the medicine being decommissioned. This will mean the medicine cannot be sold which will be costly for manufacturers.
Having reliable and effective coding technology, with a simple user operator system which minimises human errors is therefore in a manufacturer’s interests.
For expert advice on how you can ensure your coding and marking is compliant with the FMD across applications, please download our FREE guide or get in touch on enquiries@uk.interactivecoding.com.

